Natural radioactivity and radon problem
Nowadays, fear of the population of radioactivity is focused on artificial radiation sources, especially on nuclear facilities. Most people do not suspect that the greatest exposure to the population is caused by natural sources (see the following figure).
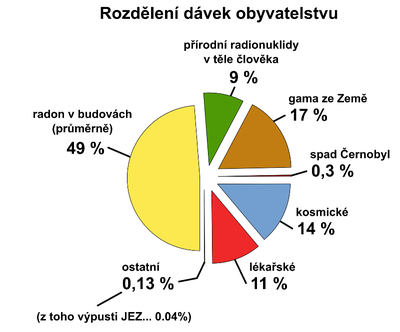
Human bodies have been always exposed to natural radiation, and to a certain extent, this exposure has been unavoidable. Some groups of the population on the Earth are exposed to radiation doses that are by one to two orders higher than the global mean value of radiation doses, and exceptionally, the doses reach the limit for deterministic radiation effects. It is surprising that attention has been devoted since the turn of the 1980s to the highest exposures to the population that are caused by indoor radon. In some houses in the Czech Republic, radon concentrations that entered from the soil were as high as the ten times the value of the limit value of the radon concentrations in uranium mines, and the annual doses of the population were more than a hundred times higher than the dose mean value in the population.
Some natural radiation sources are affected by human activities, and it is reasonable to control them. The examples are as follow: remedial measures during the construction of new buildings or the reconstruction of the existing buildings; remedial measures to reduce the exposure to the population from underground water sources with the higher concentration of natural radionuclides; and control of the natural radionuclides released to the environment during industrial activities. On this page, we want to meet you with this topic.
Natural radioactivity and its distribution
The exposure to natural sources is caused by two different sources:
- A. Cosmic radiation that impacts on the Earth, and its intensity depends on the sea level and geographical positions on the Earth.
- B. Natural radionuclides that are normally present in the environment. These nuclides can be divided into three groups according to their origin:
- Cosmogenic nuclides that are generated by the nuclear reactions during the interaction between cosmic radiation and stable isotopes, especially in the atmosphere (for example, a well-known 14C isotope is generated by the reaction 14N(n,p)14C ). The other isotopes generated are as follows: 3H, 7Be, 22Na .
-
- The original primordial nuclidesthat originated in the early stages of the universe are even now present on the Earth due to their long half-lives (>108 years) in a significant quantity (e.g. 238U, 235U, 232Th, 40K, 87Rb etc.). Many other nuclides that were early generated decayed due to their short half-lives, and in practical terms these isotopes are not now detectable.
- The original radionuclides disintegrate to the secondary radionuclides and form the decay series. From four well-known decay series, i.e., uranium-radium decay chain (starting from 238U), thorium decay chain (starting from 232Th), actinium decay chain (starting from 235U), and neptunium decay chain (starting from 237Np), we can only meet with the first three decay series in the open air.
The last two groups of natural radionuclides originate from the Earth, and these are called "terrestrial".
From the point of view of human exposure, only some natural radionuclides are important. The external exposureis mainly caused by 226Ra (or by uranium), 232Th and 40K which can be found in rocks and soil on the earth's surface (the thickness of the layer is some few tens of centimetres). The dose rate that originates from terrestrial nuclides is about 0.057 µGy/hr, (this is the mean value on the Earth), the maximum values have been measured on monazite sand in Guarapari, Brazil (up to 50 µGy/hr and in Kerala, India (about 2 µGy/hr), and on rocks with a high radium concentration in Ramsar, Iran (from 1 to 10 µGy/hr).
From the point of view of internal exposure, radon (222Rn), thoron (220Rn), and their decay products prevail. Hence, the following pages are devoted to radon.
From the point of view of internal exposure, potassium 40K. is also a significant nuclide. The potassium concentration in the human body is strictly based on the homeostatic principle, and hence the concentration is nearly stablein all persons at a level of about 55 Bq/kg, which corresponds to the annual effective dose of 0.17 mSvBecause of internal exposure, attention should be devoted to the following isotopes: radium 226Ra and 228Ra, uranium 238U and 234U, polonium 210Po and lead 210Pb. The great differences may appear in nuclide uptake (and also in corresponding doses) for individual persons or the groups of the population. With the exception of the inhalation of radon and its decay daughters that contribute to the highest doses to the population, the uptake by ingestion, in general, is much higher than that by inhalation.
From the point of view of the exposure to population, the contribution of the cosmogenic nuclides (not cosmic radiation) is negligible. It should be also mentioned that the cosmogenic nuclides (i.e. 14C and 3H) play their role in the dating of old objects.